Medpace Clinical Trials
Medpace Clinical Data Coordinator Jobs. Compared to other CROs and Pharma companies that have multiple platforms Medpace system is amazing.
 Global Cro Partner For Clinical Research Studies Medpace
Global Cro Partner For Clinical Research Studies Medpace
Medpace Jobs in Denver.

Medpace clinical trials. We provide Phase I-IV clinical development services to the biotechnology pharmaceutical and medical device industries. How feasible are they. We provide Phase I-IV clinical development services to the biotechnology pharmaceutical and medical device industries.
Medpace Medical Device specializes in medical device and diagnostic clinical development. Ad Are virtual clinical trials better. Medpace has a fully integrated system which makes it one place platform to have eTMF filing tracking of monitoring visits and other clinical trial aspects.
When factoring in bonuses and additional compensation a. Medpace is a full-service clinical contract research organization CRO. 84094 yr Software Engineer salaries - 23 salaries reported.
5375 Medpace Way Cincinnati Ohio 45227 USA Toll-free. Medpace Jobs in Columbus. 55027 yr Data Coordinator salaries - 25 salaries reported.
How feasible are they. This estimate is based upon 20 Medpace Clinical Trial Manager salary report s provided by employees or estimated based upon statistical methods. Clinical Trial Manager PM Medpace Inc.
11 rows Medpace Inc. Clinical Research Associate II salaries - 28 salaries reported. Clinical Research Associate - Senior.
Medpace Project Coordinator Jobs. Improve subject retention by offering an alternative to clinic visits. An experienced and dedicated Clinical Trial Manager leads all aspects of your study working closely with the global Medpace cross-functional project team and other vendors to seamlessly execute the trial.
Led by clinical and regulatory experts located in the US and Europe Medpace is uniquely qualified to accelerate the approval of your medical device. 39979 yr Clinical Trial Manager salaries - 24 salaries reported. Top Jobs at Medpace.
Our mission is to accelerate the global development of safe and effective medical therapeutics through its scientific and disciplined approach. We provide Phase I-IV clinical development services to the biotechnology pharmaceutical and medical device industries. Our purpose is to carry out high-quality medical research.
The Phase I Unit is located on the Medpace clinical research. We provide Phase I-IV clinical development services to the biotechnology pharmaceutical and medical device industries. At Medpace we conduct early phase clinical research studies.
Medpace is a full-service clinical contract research organization CRO. Medpace Jobs in Mason. Medpace is a full-service clinical contract research organization CRO.
The company micromanages every CRA and monitors or keeps eyes on CRAs performance so closely that your line manager will access your email account and constantly reviews your emails. Medpace is completely company interest centered. Medpace is a full-service clinical contract research organization CRO.
Medpace offers a fully-dedicated and specialized division for the design and conduct of medical device trials. Find answers to these questions. Effectively plan conduct and integrate your cardiovascular and cardiac safety trials regardless of trial size or safety demands.
Our goal is to advance medical knowledge and help bring new drugs to the public. Its managers know only Medpaces way. There are no many clinical trial projects available in Medpace.
Medpace Clinical Trial Manager Jobs. Ad Are virtual clinical trials better. Find answers to these questions.
Medpace Clinical Research Associate I Jobs. To volunteer for one of our studies you can call the Medpace Call Center at 513 366-3222 between 800 AM 800 PM ET Monday through Friday and. Director Clinical Trial Management - Cardiovascular Incentives Available Medpace Inc.
Our medical regulatory and operational experts work collaboratively with your team to design and conduct clinical research trials around the world. A Global Partner for Medical Device Clinical Studies. Medpace Entry Level Clinical Research Associate Jobs.
The typical Medpace Clinical Trial Manager salary is 88368. Medpace Regulatory Submissions Coordinator Jobs. Our mission is to accelerate the global development of safe and effective medical therapeutics through its scientific and disciplined approach.
Clinical Trial Manager salaries at Medpace can range from 49859 - 152677. Endocrine and Metabolic Unrivaled in this space Medpace can help navigate the complexities and regulatory scrutiny of. Medpace Clinical Trial Management provides critical support to ensure success and efficient delivery at every phase of global trials.
The Clinical Pharmacology Unit CPU is dedicated to the conduct of early-phase clinical pharmacology studies in normal healthy volunteers special populations and patient populations over a spectrum of diseases. Improve subject retention by offering an alternative to clinic visits. ExperiencedSenior Clinical Research Associate.
68406 yr Regulatory Submissions Coordinator salaries - 22 salaries reported. Clinical Trial Manager CTM Medpace Inc.
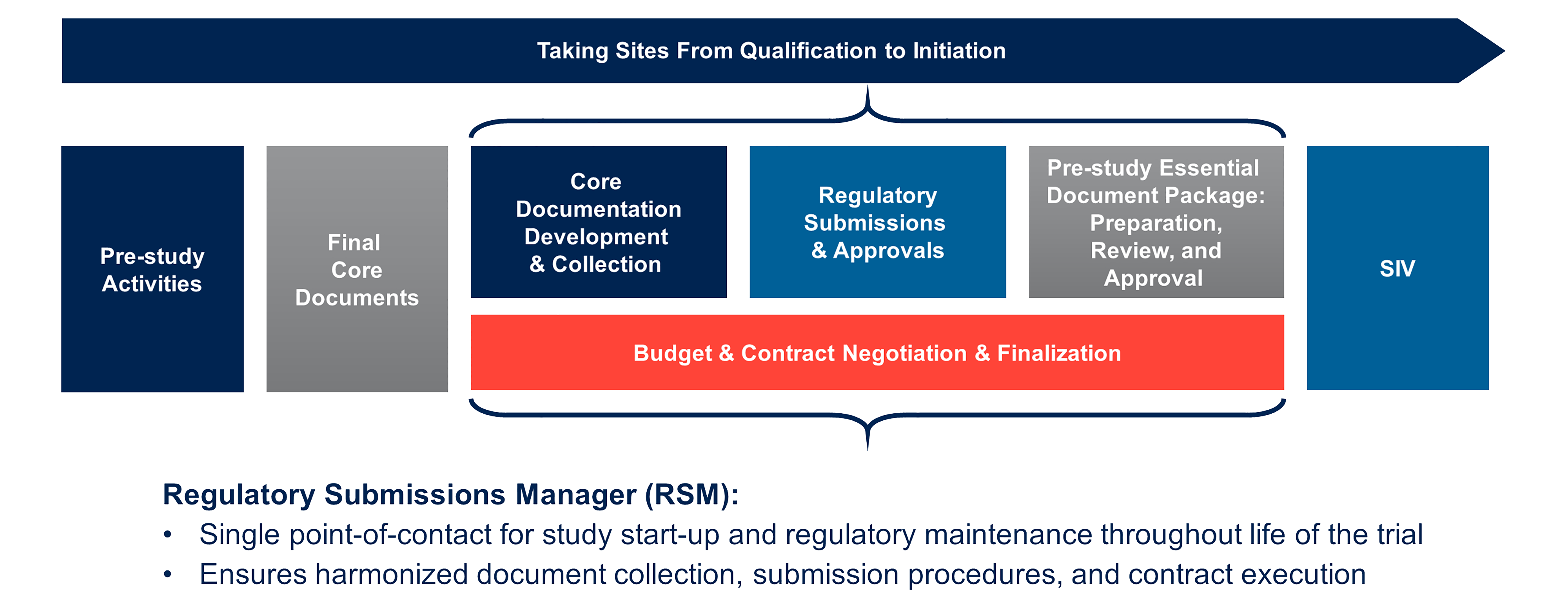 Accelerated Clinical Research Study Start Up Medpace
Accelerated Clinical Research Study Start Up Medpace
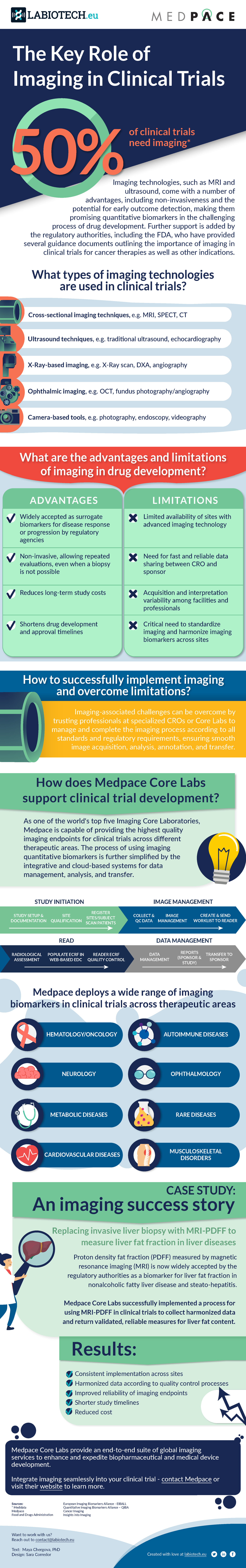 Infographic The Key Role Of Imaging In Clinical Trials Medpace
Infographic The Key Role Of Imaging In Clinical Trials Medpace
 Medidata Medpace Integrate Trial Platform
Medidata Medpace Integrate Trial Platform
 Clinical Pharmacology Unit For Phase I Trials Medpace
Clinical Pharmacology Unit For Phase I Trials Medpace
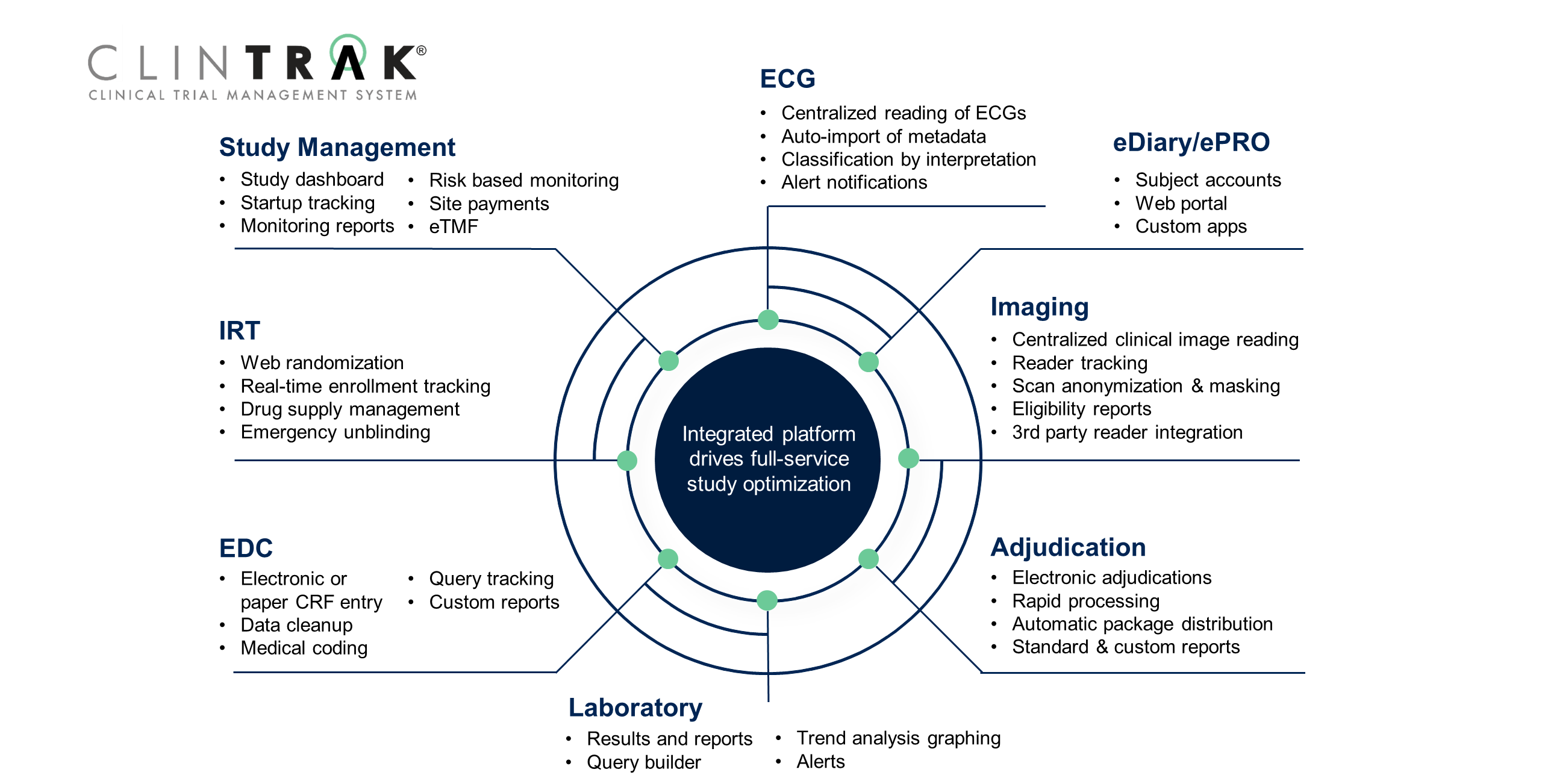 Clinical Trial Management Software Clintrak Medpace
Clinical Trial Management Software Clintrak Medpace
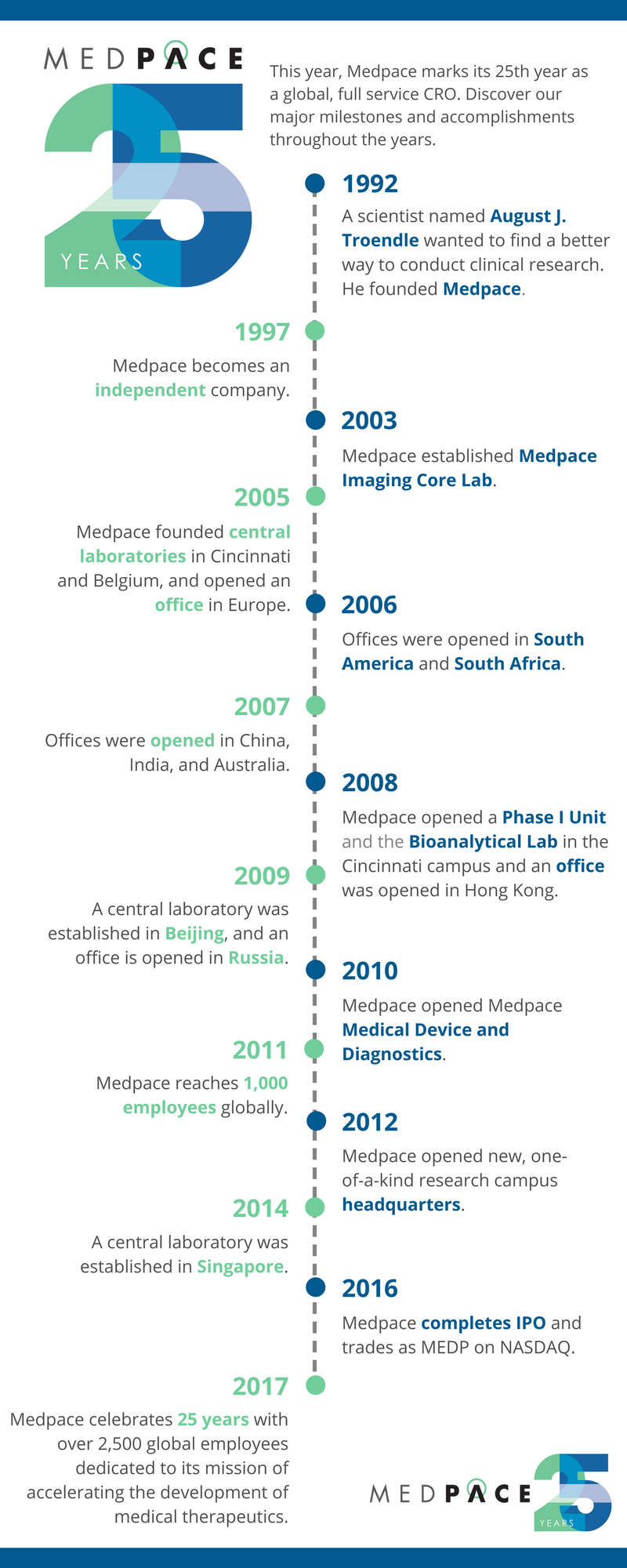 A Brief Look At The 25 Year History Of Medpace
A Brief Look At The 25 Year History Of Medpace
 Clinical Trial Management Software Services Medpace
Clinical Trial Management Software Services Medpace
 Conducting Strategic Clinical Trials In China Transcription Medpace
Conducting Strategic Clinical Trials In China Transcription Medpace
 Clinical Trial Submissions Medpace
Clinical Trial Submissions Medpace
 Global Career Opportunities In Clinical Research Medpace
Global Career Opportunities In Clinical Research Medpace
 Medical Imaging Techniques As Biomarkers In Clinical Trials
Medical Imaging Techniques As Biomarkers In Clinical Trials
 Clinical Pharmacology Unit For Phase I Trials Medpace
Clinical Pharmacology Unit For Phase I Trials Medpace
Medpace Selects Montrium Connect Edms To Improve Clinical Trial Efficiency And Support Its Continued Global Business Growth

Comments
Post a Comment